51 Billion tons of carbon are released into our atmosphere every year.
What does 1 ton of carbon look like?

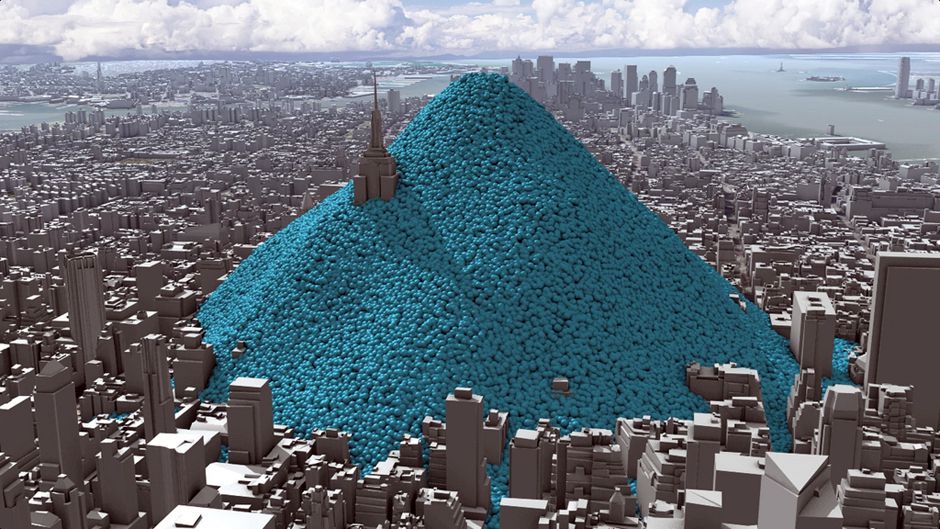
So what does 51 Billion tons look like?
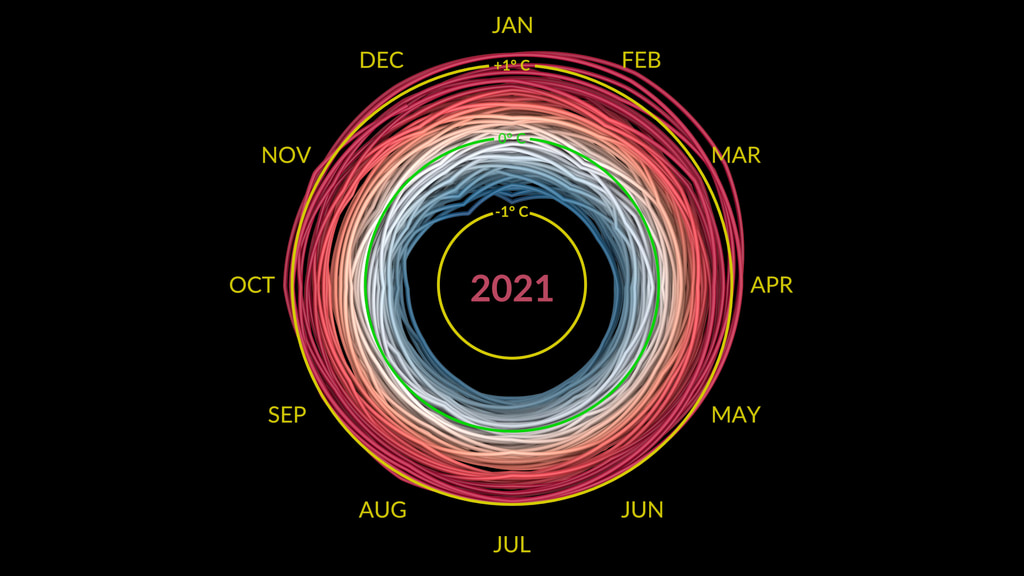
How do we know “Global Warming” is real? Because we measure it!
51 Billion tons of carbon are released into our atmosphere every year.
What does 1 ton of carbon look like?

So what does 51 Billion tons look like?

How do we know “Global Warming” is real? Because we measure it!
